Laurel Wilt Disease & Redbay Ambrosia Beetle
ENTFACT-459: Laurel Wilt Disease & Redbay Ambrosia Beetle | Download PDF
Ellen Crocker, Forest Health Extension Specialist
Tyler Dreaden, USDA Forest Service Research Plant Pathologist
Jonathan Larson, Entomology Extension Specialist
Kim Leonberger, Plant Pathology Extension Associate
Nicole Gauthier, Plant Pathology Extension Specialist
University of Kentucky College of Agriculture
Importance
Laurel wilt is a newly discovered fungal disease that presents a major threat to sassafras in Kentucky. The disease was first detected in the U.S. in 2003 and in southwestern Kentucky in 2019. Currently, laurel wilt is known to occur in eleven southeastern states bordered by Kentucky (north), North Carolina (east), Florida (south), and Texas (west). Research is ongoing to determine the impact and distribution of both the laurel wilt fungus and the redbay ambrosia beetle that carries it.
Host Range
Laurel wilt is known to affect multiple species within the laurel family (Lauraceae), which includes redbay, sassafras, spicebush, and avocado. However, in Kentucky, sassafras is the only known host and the primary species at risk for infection. Spicebush, a shrub common in forest understories and popular in landscapes, is also at risk for infection. Despite its name, mountain laurel is not in the laurel family and is not a potential host.
Symptoms
Laurel wilt symptoms include sudden wilting and discoloration of the foliage (Figure 1), followed by rapid leaf death. Initial symptoms may resemble drought or water stress. Dead sassafras leaves appear reddish-brown in color and remain attached to plants for up to 2 weeks.
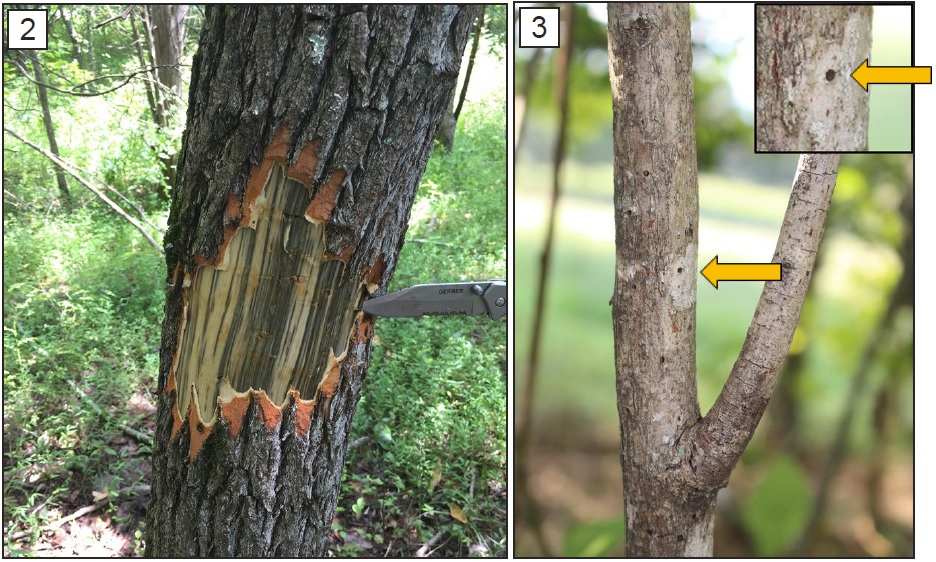
Dieback may be localized or concentrated in parts of the tree canopy, or it may affect the entire tree. Ultimately, laurel wilt results in tree death. The time in which death occurs depends upon tree species; sassafras typically die within several months of infection. However, preliminary field observations suggest that some infected trees remain alive for much longer, potentially several years. The wood underneath the bark (sapwood) may exhibit dark, streaky staining when the outer bark is cut away (Figure 2). A closer inspection of affected trunks and branches may reveal small circular holes (less than 1/10 inch in diameter; Figure 3), accompanied by thin “toothpicks” of sawdust that are caused by tunneling activity of the redbay ambrosia beetle.
Figure 1. Symptoms of laurel wilt on sassafras include sudden wilt and rapid death of branches.
Initially, leaves turn a reddish color (resembling fall leaf color) but eventually drop, leaving standing, dead trees.
Figure 2. When bark is removed, the sapwood of infected host trees exhibits dark, streaky staining.
Figure 3. Small circular holes are made by the redbay ambrosia beetle.
Figure 4. Adult redbay ambrosia beetles, which vector the laurel wilt pathogen, measure less than 1/10 inch long.
Cause & Disease Development
Laurel wilt is caused by the fungus Raffaelea lauricola, which likely overwinters in both infected wood and in beetles. Once trees become infected, the fungus rapidly colonizes sapwood and travels along waterconducting tissues (xylem). Wilt symptoms become more severe as infection spreads and water uptake is increasingly restricted, causing rapid wilt. Tree death may result from infection caused by a single pathogen introduction (e.g. a single beetle). The fungus is moved by the invasive redbay ambrosia beetle, Xyleborus glabratus (Figure 4). Ambrosia beetles native to Kentucky colonize dead or dying trees, but the redbay ambrosia beetle and some other non-natives colonize healthy, living trees. The redbay ambrosia beetle is the primary vector of the laurel wilt fungus, though other ambrosia beetle species have been reported to be contaminated with it. Redbay ambrosia beetles create tunnels (galleries) and lay eggs in the sapwood. Beetles cultivate the fungus in galleries for use as a food source for both larvae and adult beetles. Adult beetles also carry fungal spores in their mouthparts as they move to healthy trees. Disease spread occurs by natural movement of beetles, which can reach 34 miles per year, or by longer-distance spread, which occurs when infested wood is moved by humans.
The absence of insect holes does not necessarily indicate that trees are free from laurel wilt. In Kentucky, redbay ambrosia beetle populations are low enough that insect entry holes may be difficult to locate. Additionally, laurel wilt may occur in the absence of insect damage. For example, sassafras suckers (clonal tree populations) can become infected by the attached parent plant. Infection from nearby trees is also possible through root grafts. Redbay ambrosia beetles appear to be mostly attracted to large trees; as a result, those trees are generally the first ones infected. However, the abundance of disease on smaller trees suggests that tree size is not a limiting factor.
The ability of the fungus and the beetle to persist long-term in Kentucky is highly likely. Both the fungus and the beetle have been shown to survive in extreme conditions (e.g. winter cold and summer heat). Evidence also suggests that they may have survived at low levels in Kentucky before detection. Population levels in Kentucky are lower than those in other states within the beetle’s established range, and additional studies on beetle populations are ongoing.
Disease Management
No management options are currently available for laurel wilt. Thus, limiting the movement of infected wood is critical in order to limit disease incidence and spread.
- Avoid transport of wood products (e.g. firewood or debris) from infected trees or from species susceptible to laurel wilt, especially in regions where disease has been confirmed.
- Remove and destroy infected trees on site to reduce the potential for disease spread.
- If laurel wilt is suspected, contact a local county Extension office for additional assistance.
Additional Resources
- Laurel Wilt (UK Department of Forestry & Natural Resources)
http://forestry.ca.uky.edu/laurel-wilt - Laurel Wilt (Sentinel Plant Network)
http://www.sentinelplantnetwork.org/laurel-wilt - Distribution of Counties with Laurel Wilt (Forest Health, Southern Region Extension Forestry)
http://southernforesthealth.net/diseases/laurel-wilt/distribution-map - Laurel Wilt: Quick-Moving Disease with Invasive Vectors (VSC News)
http://vscnews.com/laurel-wilt-quick-moving-disease-invasive-vectors/ - Kentucky Cooperative Extension Service County Offices
http://extension.ca.uky.edu/county
July 2020
Acknowledgments
The authors would like to thank Lynne Rieske-Kinney, Forest Entomologist, University of Kentucky and David Coyle, Forest Health and Invasive Species Entomologist, Clemson University for their reviews of this publication.
Editor: Cheryl Kaiser, Extension Plant Pathology Support
Photos: Alexandra Blevins (1) and Abe Nielsen (2), Kentucky Division of Forestry; Alan Windham, University of Tennessee (3); and Joseph Benzel, Screening Aids, USDA APHIS PPQ, Bugwood.org (4)