Asian Chestnut Gall Wasp (ACGW) in Kentucky
The Asian Chestnut Gall Wasp Dryocosmus kuriphilus (Hymenoptera: Cynipidae) is stidued in the laboratory of Dr. Lynne Rieske-Kinney, Professor - Forest Entomology. Chestnut production and chestnut restoration efforts throughout the USA are threatened by the persistent spread of this exotic, invasive insect. All Castanea species are susceptible.
Symptoms and Damage

THE ASIAN CHESTNUT GALL WASP induces formation of greenish red, ½ - 1 inch round galls...

...or globular galls on actively growing chestnut shoots, petioles, or leaves.

Galls are often retained on the tree through the winter, making them especially helpful in diagnosis.
Life Cycle and Biology

ASIAN CHESTNUT GALL WASP reproduces asexually and has one generation per year. Adult females lay eggs inside buds in early summer.

These hatch soon after, and larvae feed briefly before going into diapause. Larvae overwinter within dormant buds and are impossible to detect visually, remaining inactive until buds expand the following spring.

Larval growth begins at budbreak, and induces very rapid formation of the conspicuous stem, petiole, or leaf galls.
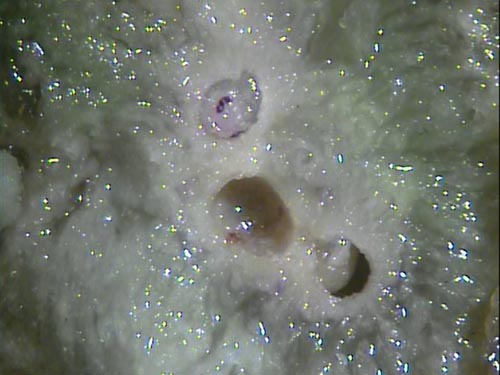
Each gall contains from one to several chambers, and each chamber contains a developing gall wasp larva. These galls provide the developing gall wasp protection throughout the larval and pupal stages. Adults emerge from galls in early summer and fly to new shoots to lay eggs for the next generation.

Once the wasps emerge, galls become woody and dry, and can remain on the tree for several years. Small leaves remain attached to the galls during the winter; these are highly visible and are useful in detecting infestations.
History and Spread
NATIVE TO CHINA, the Asian chestnut gall wasp was first reported outside its native geographic range in Japan in 1941 and on the Korean Peninsula in 1961, where it was initially managed through the use of resistant chestnut varieties.
The gall wasp was first observed in the USA in 1974 when it was reported on Chinese chestnut, C. mollissima in Fort Valley, Georgia Peach County (red) Georgia, having been imported on infested plant material. In 1976 the distribution included three adjacent counties, and the list of affected plant material included Japanese, C. crenata, and European, C. sativa, chestnuts. The geographic range expanded northward and westward, and by 1993 the host range was expanded to include chinquapin, C. pumila, and both cultivated and naturalized American chestnut, C. dentata. The range continued to expand northeasterly, reaching Meadowview, Virginia in 2001, where it readily infested hybrid crosses.
In 2002, a distinctly separate gall wasp infestation was reported in Cuyahoga County, Ohio on Chinese chestnuts in a suburban environment. This population extended east in 2005, reaching Portage County, Ohio, where it infested Chinese chestnuts cultivated for nut production. An additional isolated northerly infestation was reported on the border of Maryland and Pennsylvania in mid-2006, and in late 2006 the gall wasp was reported in Somerset County Pennsylvania, approximately equidistant between the Ohio and Maryland/Pennsylvania infestations, again on Chinese chestnuts grown for nut production. Meanwhile, the gall wasp infestation in the south expanded westward, and in 2003 reached Bowling Green, Kentucky (Warren County), where it was infesting naturalized American chestnut.
The gall wasp continues to expand its geographic range, and has since spread north to Connecticut (2011) and Massachusetts (2012) and westward to central Kentucky. It is now reported in 12 states east of the Mississippi River. D. kuriphilus is poised to continue its rapid spread through eastern North America, either through natural movement or though movement of infested plant material. Natural dispersal by flight, augmented by wind currents, can easily be sustained on naturalized American chestnut, ornamental chestnuts, and chestnuts planted for nut production. Undoubtedly the intentional movement of infested plant material poses the greatest risk, allowing the establishment of separate satellite populations well removed from contiguous populations. Interstate movement of Castanea spp. is regulated by only a few states, and the cryptic nature of the insect, lying within dormant buds for the majority of its life cycle, make the effectiveness of plant inspections questionable. Fortunately several natural enemies appear capable of exploiting D. kuriphilus, and management through natural means appears a viable option.
Natural Enemies
IN ITS NATIVE RANGE natural enemies appear to keep gall wasp populations in check; some of these natural enemies have been successfully introduced to other areas. In the eastern USA, Torymus sinensis (Hymenoptera: Torymidae) and other parasitoids were introduced for Asian chestnut gall wasp control in the mid-1970's. T. sinensis is now well established in the eastern USA. It has been manipulated for gall wasp suppression in Japan, and more recently in Europe. In addition, several native parasitoids, presumably recruited from closely related oak gallmakers, have been found in association with the Asian chestnut gall wasp. One species in particular, Ormyrus labotus (Hymenoptera: Ormyridae), has developed the ability to exploit the gall wasp, and may contribute to gall wasp population suppression in some situations.
Management
INSECTICIDES ARE INEFFECTIVE in suppressing gall wasp populations, due in part to the nature of gall tissue. The introduced parasitoid T. sinensis has expanded its geographic range in the USA along with expanding gall wasp populations. T. sinensis produces one generation per year; adults emerge in early spring and parasitize newly developing galls. The parasite overwinters in vacated galls as pupae and larvae, emerging in spring in time to parasitize newly forming galls. This synchrony with the D. kuriphilus life cycle contributes to the efficacy of T. sinensis as a natural enemy, and may explain why T. sinensis acts as a specialist. Anecdotal evidence suggests that additional parasitoids from the 1970's releases may also have established in the USA; results are pending.
Unlike T. sinensis, the native O. labotus is a generalist that produces multiple generations per year. It emerges from chestnut galls between July and August and is absent for the remainder of the year, indicating that it requires additional hosts throughout the remaining summer and winter months following its emergence from chestnut. O. labotus also emerges from dead winter galls which do not contain living D. kuriphilus, and only contain T. sinensis, indicating an antagonistic interaction between the native and introduced parasitoids. This suggests that in addition to being a primary parasitoid of D. kuriphilus, O. labotus is hyperparasitizing T. sinensis, and may suppress T. sinensis populations in some locations.
O. labotus is a common parasitoid of many oak-galling cynipids, and is more abundant in chestnut found in forest mosaics with the greatest woody plant diversity and oak component, providing it with abundant alternate hosts. In contrast, chestnuts growing in settings with lower plant diversity tend to have a natural enemy complex dominated by the introduced T. sinensis.
Our study suggests competitive interactions between these native and introduced parasitoids that vary among habitat types. T. sinensis tends to be the primarily parasitoid where chestnut is numerically dominant, whereas T. sinensis and O. labotus may both be common in more vegetatively diverse sites. Collectively we’re working to improve our understanding of the interactions between an invasive insect, its introduced parasitoid, and native parasitoid recruitment which will lead to development of Asian chestnut gall wasp management strategies in cultivated chestnut.
Resources
- Rieske, L.K. 2007. Success of an exotic gallmaker, Dryocosmus kuriphilus, on chestnut in the USA: a historical account. Bulletin of the European and Mediterranean Plant Protection Organization (EPPO Bulletin) 37, 172-174.
- Can Our Chestnut Survive Another Invasion? Lynne K. Rieske and W. Rodney Cooper, Proceedings of the 17th Central Hardwood Forest Conference GTR-NRS-P-78 (2011)
